
Chemistry, 20.04.2021 22:40 alyssaboosiefkes
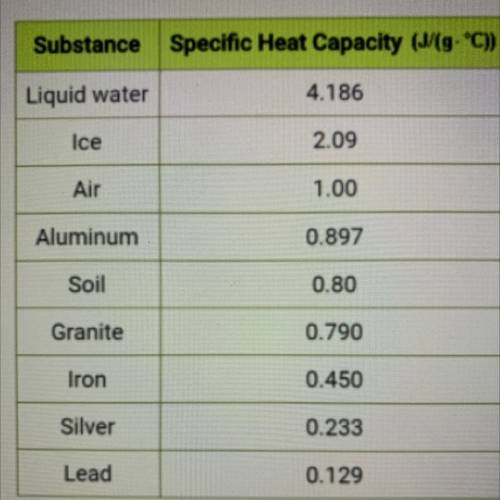
Use the specific heat value from the table at left to calculate the amount
of energy in Joules required to raise the temperature of 5.08 g of
aluminum by 13.20 °C. Do not record units in your answer. Round to
the tenths place or further.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 13:30
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1
You know the right answer?
Use the specific heat value from the table at left to calculate the amount
of energy in Joules req...
Questions




Social Studies, 08.04.2020 22:26


Mathematics, 08.04.2020 22:26




English, 08.04.2020 22:26

History, 08.04.2020 22:26



Mathematics, 08.04.2020 22:26


Mathematics, 08.04.2020 22:26

Social Studies, 08.04.2020 22:26






