
Chemistry, 20.04.2021 21:30 nancieabreu4491
If 10.0 Moles Of Hydrochloric Acid Are Reacted With Excess Aluminum, How Many Moles Of Aluminum Chloride Could Be Formed? Explain
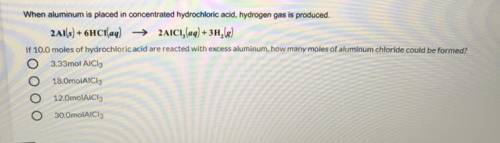

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
If 10.0 Moles Of Hydrochloric Acid Are Reacted With Excess Aluminum, How Many Moles Of Aluminum Chlo...
Questions








Biology, 08.10.2019 04:10

Mathematics, 08.10.2019 04:10




Biology, 08.10.2019 04:10

Arts, 08.10.2019 04:10


English, 08.10.2019 04:10

History, 08.10.2019 04:10


Social Studies, 08.10.2019 04:10



