
Chemistry, 20.04.2021 09:30 zahnjoey4661
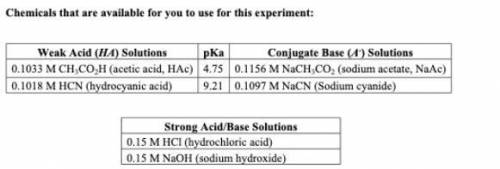
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its conjugate base that would create a buffer that best fits your protein. Would you expect for your buffer to have more acid or more base?
My assigned protein is Xylanase and has an optimum pH of 5.5.
2. Buffers are used to the inhibit the change of pH upon the addition of strong acids and bases. If you were to add 0.1 M HCl to your buffer, would you expect the pH to change? If so, would the pH increase or decrease? What would happen if 0.1M NaOH were to be added instead?
3. Keeping your buffer composition from question 1 in mind, would you expect to use a larger volume of HCl or NaOH to change the pH of the buffer solution by one unit? Explain.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its co...
Questions

Mathematics, 16.12.2019 21:31


Health, 16.12.2019 21:31

Chemistry, 16.12.2019 21:31

History, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31


Business, 16.12.2019 21:31

History, 16.12.2019 21:31


Biology, 16.12.2019 21:31

Mathematics, 16.12.2019 21:31




Mathematics, 16.12.2019 21:31



History, 16.12.2019 21:31

Social Studies, 16.12.2019 21:31



