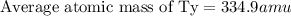Chemistry, 19.09.2019 08:00 loveniasummer71
Tyserium (ty) consists of two isotopes. one isotope has a mass of 331 amu and is 35.0 % abundant. the other isotope is 337 amu and is 65.0 % abundant. what is the mass of ty as it appears on the periodic table?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
Tyserium (ty) consists of two isotopes. one isotope has a mass of 331 amu and is 35.0 % abundant. th...
Questions

Geography, 26.03.2020 22:58

Mathematics, 26.03.2020 22:58

Mathematics, 26.03.2020 22:58






Mathematics, 26.03.2020 22:58



Mathematics, 26.03.2020 22:58

Mathematics, 26.03.2020 22:58

Mathematics, 26.03.2020 22:58




Biology, 26.03.2020 22:58


Mathematics, 26.03.2020 22:58

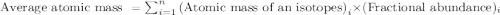 .....(1)
.....(1)![\text{Average atomic mass of Ty}=[(331\times 0.35)+(337\times 0.65)]](/tpl/images/0242/4851/befca.png)