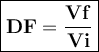Chemistry, 02.09.2019 00:30 4300402428
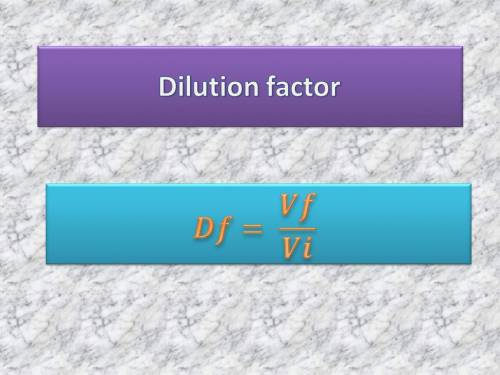
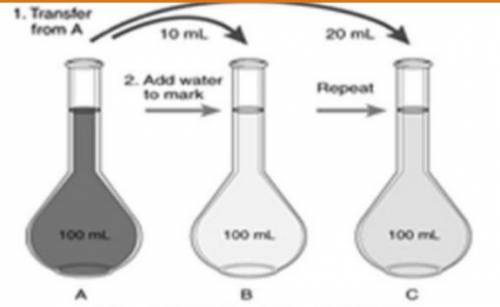
If the concentration of the stock solution is 25 m, what would be the dilution factor and concentration of sample ‘c’ with respect to original stock solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1


Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
If the concentration of the stock solution is 25 m, what would be the dilution factor and concentrat...
Questions

English, 29.08.2019 11:00

History, 29.08.2019 11:00

Physics, 29.08.2019 11:00



Chemistry, 29.08.2019 11:10

Mathematics, 29.08.2019 11:10


Biology, 29.08.2019 11:10

Mathematics, 29.08.2019 11:10




Mathematics, 29.08.2019 11:10


Biology, 29.08.2019 11:10

Social Studies, 29.08.2019 11:10



Arts, 29.08.2019 11:10