Chemistry, 29.09.2019 18:30 andrejr0330jr
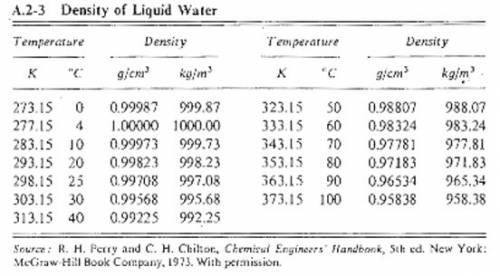
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 14°c instead. how is the mass of the 50.0 ml of distilled water you measured at 14°c different from the mass of 50.0 ml of distilled water at 20°c?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature...
Questions

Computers and Technology, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10


Mathematics, 09.03.2022 16:10

Social Studies, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10

Physics, 09.03.2022 16:10

English, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10


Mathematics, 09.03.2022 16:10

History, 09.03.2022 16:10


Mathematics, 09.03.2022 16:10

Mathematics, 09.03.2022 16:10

Physics, 09.03.2022 16:10


Mathematics, 09.03.2022 16:10