Which is the next logical step in balancing the given equation?
cs2(l) + cl2(g) ccl4(l) + s2c...

Chemistry, 23.01.2020 04:31 lovecats12
Which is the next logical step in balancing the given equation?
cs2(l) + cl2(g) ccl4(l) + s2cl2(l)
place the coefficient 2 in front of sulfur dichloride (s2cl2).
place the coefficient 3 in front of the chlorine molecule.
leave the equation alone as it is already balanced.
place the coefficient 4 in front of carbon disulfide(cs2).
place the coefficient 2 in front of carbon tetrachloride(ccl4).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
Questions


English, 27.08.2019 21:30


English, 27.08.2019 21:30

Physics, 27.08.2019 21:30

English, 27.08.2019 21:30

Mathematics, 27.08.2019 21:30


Health, 27.08.2019 21:30


Chemistry, 27.08.2019 21:30


History, 27.08.2019 21:30



Social Studies, 27.08.2019 21:30

Social Studies, 27.08.2019 21:30


Health, 27.08.2019 21:30

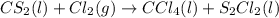
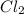 molecule.
molecule.



