
Chemistry, 18.12.2019 06:31 ajyoung3142005oztpya
You are dating moon rocks based on their proportions of uranium-238 (half-life of about 4.5 billion years) and its ultimate decay product, lead.
1. find the age for a rock for which you determine that 55% of the original uranium-238 remains, while the other 45% has decayed into lead.
2. find the age for a rock for which you determine that 68% of the original uranium-238 remains, while the other 32% has decayed into lead.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
You are dating moon rocks based on their proportions of uranium-238 (half-life of about 4.5 billion...
Questions

History, 19.01.2020 06:31

Biology, 19.01.2020 06:31


Mathematics, 19.01.2020 06:31


Biology, 19.01.2020 06:31


Mathematics, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31

Biology, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31


Mathematics, 19.01.2020 06:31


Biology, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31


Mathematics, 19.01.2020 06:31

![-kt = ln[\frac{FinalAmount}{InitialAmount}]](/tpl/images/0423/9056/e9d33.png)
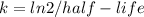
![t = - \frac{4.5}{ln2} \times ln[\frac{0.55}{1}]](/tpl/images/0423/9056/ff6e0.png)
![t = - \frac{4.5}{ln2} \times ln[\frac{0.63}{1}]](/tpl/images/0423/9056/f1017.png)


