
Chemistry, 24.08.2019 23:20 eeromaki1321
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to react with 0.536 moles of li.
the number of moles of li required to make 46.4 g of li3n.
the mass in grams of li3n produced from 3.65 g li.
the number of moles of lithium needed to react with 7.00 grams of n2.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3


Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
Questions


Mathematics, 10.02.2021 22:20

History, 10.02.2021 22:20


Chemistry, 10.02.2021 22:20

Mathematics, 10.02.2021 22:20

Mathematics, 10.02.2021 22:20




Mathematics, 10.02.2021 22:20




English, 10.02.2021 22:20


Mathematics, 10.02.2021 22:20

Biology, 10.02.2021 22:20


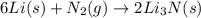
 needed to react with 0.536 moles of Li.
needed to react with 0.536 moles of Li.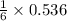 moles of
moles of ![N_2[tex] gas needed:[tex]=28 g/mol\times 0.0893 mol=2.5004 g](/tpl/images/0194/9373/6ce3e.png)

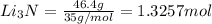
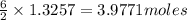
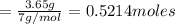
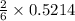 that is 0.1738 moles of
that is 0.1738 moles of 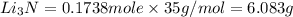
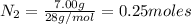
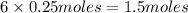 of lithium
of lithium

