
Chemistry, 16.01.2020 23:31 PeachyPeaches1489
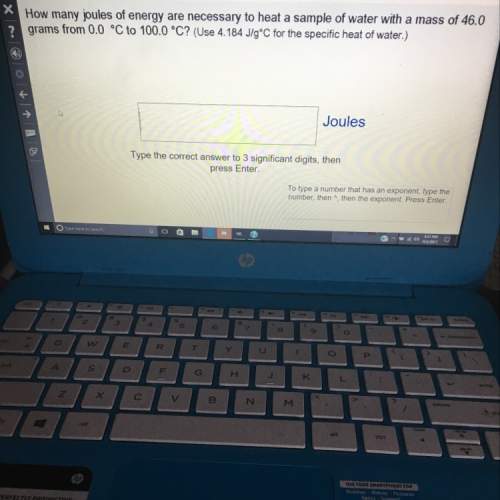
How many joules of energy are necessary to heat a sample of water with a mass of 46.0 grams from 0.0 celsius to 100.0 celsius? (use 4.184 j/g celsius for the specific heat of water.)
type the correct answer to 3 significant digits. if the answer has an exponent, type the number, then ^, then the exponent.
(the question is on the picture.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
How many joules of energy are necessary to heat a sample of water with a mass of 46.0 grams from 0.0...
Questions

Mathematics, 31.07.2019 11:00


Social Studies, 31.07.2019 11:00






Business, 31.07.2019 11:00


History, 31.07.2019 11:00


History, 31.07.2019 11:00


Biology, 31.07.2019 11:00


Biology, 31.07.2019 11:00

Mathematics, 31.07.2019 11:00






