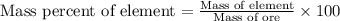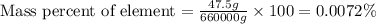Chemistry, 01.09.2019 18:30 keishah577
In 1928, 47.5 g of a new element was isolated from 660 kg of the ore molybdenite. the percent by mass of this element in the ore was:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
You know the right answer?
In 1928, 47.5 g of a new element was isolated from 660 kg of the ore molybdenite. the percent by mas...
Questions



Mathematics, 03.12.2021 02:40


Physics, 03.12.2021 02:40

Mathematics, 03.12.2021 02:40


Law, 03.12.2021 02:40

Mathematics, 03.12.2021 02:40

Spanish, 03.12.2021 02:40

Physics, 03.12.2021 02:40



English, 03.12.2021 02:40


Mathematics, 03.12.2021 02:40




Health, 03.12.2021 02:40