
Chemistry, 22.11.2019 12:31 natalie2sheffield
15 which statement describes the particles of an ideal gas, based on the kinetic molecular theory?
(1) the motion of the gas particles is orderly and circular.
(2) the gas particles have no attractive forces between them.
(3) the gas particles are larger than the distances separating them.
(4) as the gas particles collide, the total energy of the system decreases.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Imagine that you own a property that is exactly 2.2 acres large. you want to sell your property, but your realtor tells you that you cannot sell your land by the acre. in order to sell your land you need to determine the area you own in units of square meters? given that there are 1.6 kilometers in 1 mile and 640 acres in 1 square mile, what is the area of land that you own in square meters square meters?
Answers: 2

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

You know the right answer?
15 which statement describes the particles of an ideal gas, based on the kinetic molecular theory? <...
Questions

Mathematics, 03.03.2021 20:10

History, 03.03.2021 20:10




Mathematics, 03.03.2021 20:10


Social Studies, 03.03.2021 20:10


Business, 03.03.2021 20:10

Mathematics, 03.03.2021 20:10

Mathematics, 03.03.2021 20:10


History, 03.03.2021 20:10


Mathematics, 03.03.2021 20:10

English, 03.03.2021 20:10


English, 03.03.2021 20:10

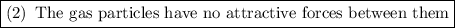 .
.



