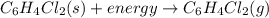Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)...

Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)
which statement describes this change?
(1) it is endothermic, and entropy decreases.
(2) it is endothermic, and entropy increases.
(3) it is exothermic, and entropy decreases.
(4) it is exothermic, and entropy increases.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
Questions

Social Studies, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Arts, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Chemistry, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Biology, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

English, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

Social Studies, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01

English, 14.09.2020 19:01

Mathematics, 14.09.2020 19:01