
Chemistry, 23.08.2019 08:50 desderievelasquez
Asample of ca(oh)2 is considered to be an arrhenius base because it dissolves in water to yield
a. ca2+ ions as the only positive ions in solution
b. h3o+ ions as the only positive ions in solution
c. oh– ions as the only negative ions in solution
d. h– ions as the only negative ions in solution

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Asample of ca(oh)2 is considered to be an arrhenius base because it dissolves in water to yield
Questions

Social Studies, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00




Mathematics, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00


English, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00


Mathematics, 31.03.2021 18:00



English, 31.03.2021 18:00

Mathematics, 31.03.2021 18:00



Mathematics, 31.03.2021 18:00

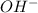 ions as the only negative ions in solution
ions as the only negative ions in solution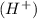 and a bases is a substance that ionizes in the water to give hydroxide ion
and a bases is a substance that ionizes in the water to give hydroxide ion 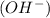 .
.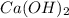 is considered to be an Arrhenius base because when it dissolves in the water to yield
is considered to be an Arrhenius base because when it dissolves in the water to yield 

