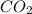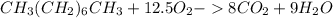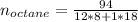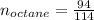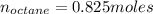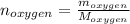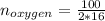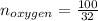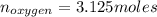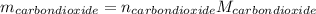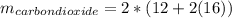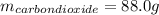Chemistry, 30.08.2019 16:20 deadpoolcorvettehats
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 94. g of octane is mixed with 100. g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
Liquid octane ch3ch26ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and...
Questions



Mathematics, 09.04.2020 21:01

Mathematics, 09.04.2020 21:01

Mathematics, 09.04.2020 21:01




English, 09.04.2020 21:02

Biology, 09.04.2020 21:02

English, 09.04.2020 21:02

Mathematics, 09.04.2020 21:02

Biology, 09.04.2020 21:02


Biology, 09.04.2020 21:02


Mathematics, 09.04.2020 21:02

Mathematics, 09.04.2020 21:02


Biology, 09.04.2020 21:02