
Chemistry, 16.09.2019 11:00 erikacastro2421
How many ml of concentrated nitric acid (hno3, 16.0 m) should be diluted with water in order to make 2.00 l of 2.00 m solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
How many ml of concentrated nitric acid (hno3, 16.0 m) should be diluted with water in order to make...
Questions

Mathematics, 20.09.2019 15:30

History, 20.09.2019 15:30

English, 20.09.2019 15:30

History, 20.09.2019 15:30



History, 20.09.2019 15:30



Physics, 20.09.2019 15:50


Mathematics, 20.09.2019 15:50


Business, 20.09.2019 15:50




Mathematics, 20.09.2019 15:50


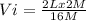
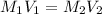
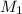 = molarity of stock
= molarity of stock 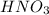 solution = 16 M
solution = 16 M
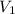 = volume of stock
= volume of stock 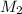 = molarity of diluted
= molarity of diluted 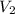 = volume of diluted
= volume of diluted 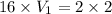
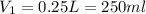 (Conversion factor: 1L=1000ml)
(Conversion factor: 1L=1000ml)

