
Chemistry, 01.09.2019 02:30 nulledcracker12
Consider the balanced chemical equation that follows. you are asked to determine how many moles of water you can produce from 4.0 mol of hydrogen and excess oxygen. (excess oxygen means that so much oxygen is available it will not run out.) which of the numbers that appear in the balanced chemical equation below are used to perform this calculation? 2h2(g)+o2(g)→2h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Consider the balanced chemical equation that follows. you are asked to determine how many moles of w...
Questions

Mathematics, 07.10.2021 20:50

Physics, 07.10.2021 20:50


Mathematics, 07.10.2021 20:50

English, 07.10.2021 20:50

History, 07.10.2021 20:50


Computers and Technology, 07.10.2021 20:50

Mathematics, 07.10.2021 20:50

Mathematics, 07.10.2021 20:50

Physics, 07.10.2021 20:50

Mathematics, 07.10.2021 20:50

History, 07.10.2021 20:50


World Languages, 07.10.2021 20:50


Advanced Placement (AP), 07.10.2021 20:50


Mathematics, 07.10.2021 20:50

Mathematics, 07.10.2021 20:50

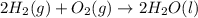
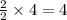 moles of water
moles of water

