
Chemistry, 26.08.2019 18:30 emmaccanttlon
What is the [h+] of an hcl solution if the ph is measured to be 6? select one: a. 1 x 10-7 m b. 1 x 10-6 m c. 6 x 10-6 m d. 8 x 10-1 m

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

You know the right answer?
What is the [h+] of an hcl solution if the ph is measured to be 6? select one: a. 1 x 10-7 m b. 1...
Questions

Mathematics, 17.09.2019 01:30



World Languages, 17.09.2019 01:30



Mathematics, 17.09.2019 01:30


Physics, 17.09.2019 01:30

History, 17.09.2019 01:30


Social Studies, 17.09.2019 01:30


Mathematics, 17.09.2019 01:30





History, 17.09.2019 01:30

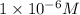
![pH=-\log [H^+]](/tpl/images/0199/9478/37e81.png)
![6=-\log [H^+]](/tpl/images/0199/9478/be8fc.png)
![[H^+]=1\times 10^{-6}M](/tpl/images/0199/9478/67fdd.png)


