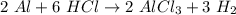Chemistry, 20.08.2019 23:50 liyahheadhigh
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6 moles of hydrochloric acid (hcl)? a. 2 moles of alcl2 and 1 mole of h2 b. 3 moles of alcl3 and 2 moles of h2 c. 2 moles of alcl3 and 3 moles of h2 d. 3 moles of alcl2 and 3 moles of h2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 08:00
Straightforward questions answered in the powerpoint slidesreaction: heating the starting materials under refluxwhat does it mean to heat under reflux? why do we choose water as the reflux solvent? what are boiling chips used for? why do we put a condenser on top of the reaction? why do we add heat and let the reaction stir for 30 minutes? why do we add sulfuric acid to the reaction after it cools as opposed to when it’s still hot? separation: filtration of precipitatewhy don’t we do an aqueous and organic extraction in the separatory funnel? why do you rinse the salicylic acid on the filter with ice cold water? purification: recrystallization of salicylic acid (no hot filtration needed)what is the difference in the amount of room temperature water vs. boiling water needed to dissolve the salicylic acid (assume a 1.2 gram yield of salicylic acid)? remember, in the lab if you need x ml of boiling water to dissolve a solid, then you should add a little more (definitely no more than 1.5 times the theoretical amount) to ensure it doesn’t recrystallize prematurely.analysis: melting point of salicylic acidwhat can you conclude if the melting point of the salicylic acid you just synthesized is 152-155oc and the 1: 1 mix of your product and “synthetic” salicylic acid is 151-154oc?
Answers: 1

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6...
Questions




Mathematics, 26.04.2021 23:00

Mathematics, 26.04.2021 23:00



Mathematics, 26.04.2021 23:00




Advanced Placement (AP), 26.04.2021 23:00

Mathematics, 26.04.2021 23:00



Mathematics, 26.04.2021 23:00


Mathematics, 26.04.2021 23:00

Mathematics, 26.04.2021 23:00

English, 26.04.2021 23:00