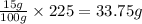Chemistry, 01.09.2019 11:30 hannah2757
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding 39.0g of potassium sulfate to 225g water, carefully heating the solution, and cooling it to 40 celsius. a homogeneous solution is obtained. is this solution saturated, unsaturated, or supersaturated? the beaker is shaken and precipitation occurs. how many grams of potassium sulfate would you except to crystallize out?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
Potassium sulfate has a solubility of 15g/100g water at 40 celsius. a solution is prepared by adding...
Questions

Mathematics, 18.11.2019 12:31

Health, 18.11.2019 12:31

Mathematics, 18.11.2019 12:31


Mathematics, 18.11.2019 12:31

Mathematics, 18.11.2019 12:31


Mathematics, 18.11.2019 12:31



Computers and Technology, 18.11.2019 12:31





Chemistry, 18.11.2019 12:31


Mathematics, 18.11.2019 12:31

History, 18.11.2019 12:31