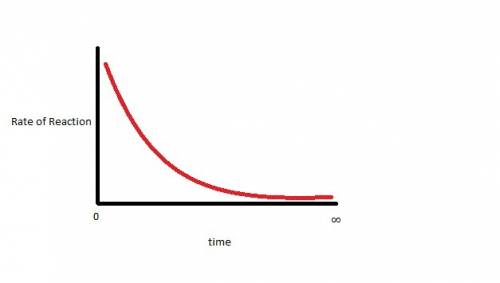
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed in an evacuated glass container, the closed system eventually reaches dynamic equilibrium. which statement describes the graph of the rate of the forward reaction over time? it starts high and gradually decreases until it levels out above zero. it starts high and gradually decreases until it reaches a rate of zero. it starts low and gradually increases until it levels out at a rate above zero. it starts low and gradually increases until it reaches a maximum rate.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Dinitrogen tetroxide decomposes to produce nitrogen dioxide gas. when dinitrogen tetroxide is sealed...
Questions






Computers and Technology, 18.09.2019 21:00



Mathematics, 18.09.2019 21:00










Mathematics, 18.09.2019 21:00