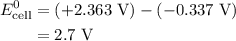Chemistry, 17.09.2019 07:30 nuggetslices
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reactions at 25 °c. write the equation for the cell reaction that occurs under standard-state conditions and write the line notation for the cell.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Calculate the standard emf of a cell that uses the mg/mg2+ and cu/cu2+ half-cell reactions at 25 °c....
Questions

Social Studies, 10.07.2019 17:30

Geography, 10.07.2019 17:30


Physics, 10.07.2019 17:30


History, 10.07.2019 17:30

Mathematics, 10.07.2019 17:30



Mathematics, 10.07.2019 17:30



Physics, 10.07.2019 17:30







Mathematics, 10.07.2019 17:30


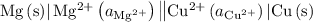
 .
.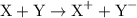
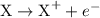

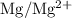 is
is 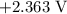 .
.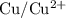 is
is 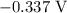 .
.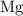 has higher oxidation potential thus the oxidation of
has higher oxidation potential thus the oxidation of 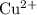 takes place at cathode.
takes place at cathode.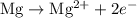 ......(1)
......(1)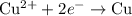 ......(2)
......(2)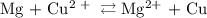 ......(3)
......(3)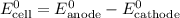 ......(5)
......(5)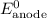 and
and 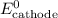 in equation (5).
in equation (5).