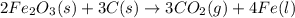Chemistry, 31.08.2019 19:10 noahdeno200010125
The balanced equation for the reaction occurring when iron(iii) oxide, a solid, is reduced with pure carbon to produce carbon dioxide and molten iron is

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
The balanced equation for the reaction occurring when iron(iii) oxide, a solid, is reduced with pure...
Questions

Mathematics, 26.08.2021 15:20

Social Studies, 26.08.2021 15:20





English, 26.08.2021 15:20

Arts, 26.08.2021 15:30

Mathematics, 26.08.2021 15:30

Mathematics, 26.08.2021 15:30

Chemistry, 26.08.2021 15:30

SAT, 26.08.2021 15:30

Chemistry, 26.08.2021 15:30

Mathematics, 26.08.2021 15:30

Mathematics, 26.08.2021 15:30

World Languages, 26.08.2021 15:30

Mathematics, 26.08.2021 15:30

Computers and Technology, 26.08.2021 15:30

History, 26.08.2021 15:30