
Chemistry, 24.12.2019 08:31 rwbrayan8727
The compound ha is an acid that is soluble in water which of the beakers in the picture shows ha behaving as a weak acid in water?
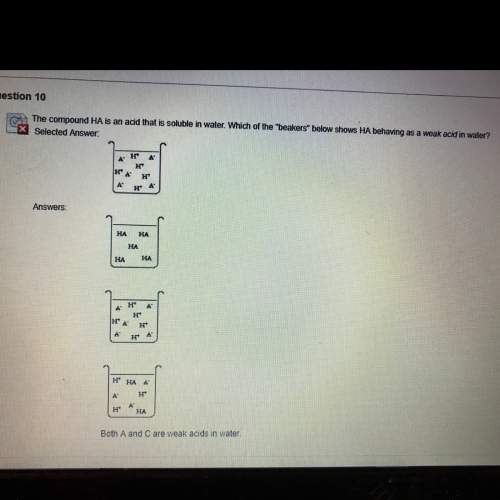

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
The compound ha is an acid that is soluble in water which of the beakers in the picture shows ha beh...
Questions

Mathematics, 02.09.2021 08:20

Mathematics, 02.09.2021 08:20

Mathematics, 02.09.2021 08:20

Mathematics, 02.09.2021 08:20

Mathematics, 02.09.2021 08:20


Mathematics, 02.09.2021 08:20

Mathematics, 02.09.2021 08:20



History, 02.09.2021 08:20


Chemistry, 02.09.2021 08:20

Biology, 02.09.2021 08:20


Mathematics, 02.09.2021 08:20


Computers and Technology, 02.09.2021 08:20


Physics, 02.09.2021 08:20



