
Chemistry, 23.09.2019 21:00 alyxkellar06
For the reaction c2h6 (g) → c2h4 (g) + h2 (g) δh° is +137 kj/mol and δs° is +120 j/k ∙ mol. this reaction is question 10 options: spontaneous only at high temperature spontaneous at all temperatures spontaneous only at low temperature nonspontaneous at all temperatures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3
You know the right answer?
For the reaction c2h6 (g) → c2h4 (g) + h2 (g) δh° is +137 kj/mol and δs° is +120 j/k ∙ mol. this rea...
Questions

Chemistry, 07.01.2021 08:40

Arts, 07.01.2021 08:40

Mathematics, 07.01.2021 08:40

Social Studies, 07.01.2021 08:40


Business, 07.01.2021 08:40


Biology, 07.01.2021 08:40

Mathematics, 07.01.2021 08:40

Chemistry, 07.01.2021 08:40

Physics, 07.01.2021 08:40

Spanish, 07.01.2021 08:40


Mathematics, 07.01.2021 08:40

Mathematics, 07.01.2021 08:40

Chemistry, 07.01.2021 08:40

Mathematics, 07.01.2021 08:40



Social Studies, 07.01.2021 08:40

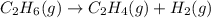
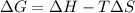
 = Gibbs free energy
= Gibbs free energy  = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = enthalpy change = endothermic = +137 KJ
= enthalpy change = endothermic = +137 KJ 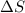 = entropy change = +120 J/K
= entropy change = +120 J/K 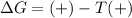
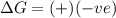
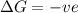 when
when  has more value than
has more value than 

