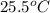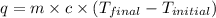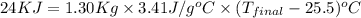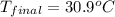Complete combustion of a 0.600-g sample of a compound in a bomb calorimeter releases 24.0 kj of heat. the bomb calorimeter has a mass of 1.30 kg and a specific heat of 3.41 j/(gi°c). if the initial temperature of the calorimeter is 25.5°c, what is its final temperature? use mc026-1.jpg. 30.9°c 34.5°c 44.0°c 51.5°c

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1


Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Complete combustion of a 0.600-g sample of a compound in a bomb calorimeter releases 24.0 kj of heat...
Questions


Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Health, 03.03.2021 01:00


Mathematics, 03.03.2021 01:00

Advanced Placement (AP), 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

English, 03.03.2021 01:00


English, 03.03.2021 01:00

Health, 03.03.2021 01:00





Mathematics, 03.03.2021 01:00

Social Studies, 03.03.2021 01:00

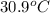
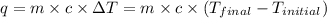
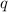 = heat released = 24 KJ
= heat released = 24 KJ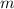 = mass of bomb calorimeter = 1.30 Kg
= mass of bomb calorimeter = 1.30 Kg
 = specific heat =
= specific heat = 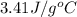
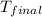 = final temperature = ?
= final temperature = ?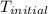 = initial temperature =
= initial temperature =