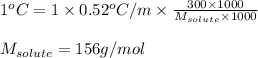Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
What is the approximate molar mass of a molecular solute if 300 g of the solute in 1000 g of water c...
Questions

Biology, 18.08.2019 10:30

Biology, 18.08.2019 10:30

Business, 18.08.2019 10:30

History, 18.08.2019 10:30

Chemistry, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30

Biology, 18.08.2019 10:30

Social Studies, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30


Mathematics, 18.08.2019 10:30

Social Studies, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30

Chemistry, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30

Mathematics, 18.08.2019 10:30




Social Studies, 18.08.2019 10:30

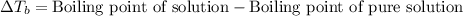
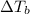 = ? °C
= ? °C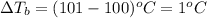
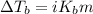
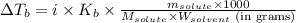
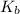 = molal boiling point elevation constant = 0.52°C/m.g
= molal boiling point elevation constant = 0.52°C/m.g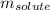 = Given mass of solute = 300 g
= Given mass of solute = 300 g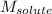 = Molar mass of solute = ?
= Molar mass of solute = ?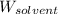 = Mass of solvent (water) = 1000 g
= Mass of solvent (water) = 1000 g