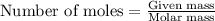Chemistry, 11.10.2019 18:40 brydenskl814
Asample of ethanol (c2h6o) has a mass of 0.2301 g. complete combustion of this sample causes the temperature of a bomb calorimeter to increase by 1.33°c. the calorimeter has a mass of 2.000 kg and a specific heat of 2.45 j/g•°c. how many moles of ethanol are present in the sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Asample of ethanol (c2h6o) has a mass of 0.2301 g. complete combustion of this sample causes the tem...
Questions

Physics, 26.02.2020 19:30

Chemistry, 26.02.2020 19:30


Mathematics, 26.02.2020 19:31



Mathematics, 26.02.2020 19:31



Mathematics, 26.02.2020 19:31

Health, 26.02.2020 19:31





Computers and Technology, 26.02.2020 19:31



English, 26.02.2020 19:31

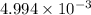 moles
moles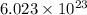 of particles.
of particles.