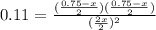Chemistry, 29.09.2019 15:30 rebecca7415
At a certain temperature the equilibrium constant, kc, equals 0.11 for the reaction: 2 icl(g)? i2(g) + cl2(g). what is the equilibrium concentration of icl if 0.75 mol of i2 and 0.75 mol of cl2 are initially mixed in a 2.0-l flask?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1
You know the right answer?
At a certain temperature the equilibrium constant, kc, equals 0.11 for the reaction: 2 icl(g)? i2(g...
Questions



Mathematics, 29.06.2019 22:00

Chemistry, 29.06.2019 22:00

Mathematics, 29.06.2019 22:00

Mathematics, 29.06.2019 22:00

Biology, 29.06.2019 22:00

History, 29.06.2019 22:00

Mathematics, 29.06.2019 22:00

English, 29.06.2019 22:00




Mathematics, 29.06.2019 22:00


Social Studies, 29.06.2019 22:00