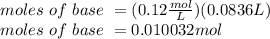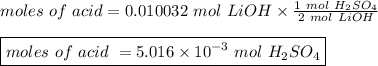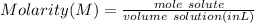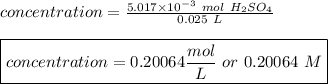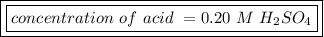Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
The titration of 25.0 ml of an unknown concentration h2so4 solution requires 83.6 ml of 0.12 m lioh...
Questions

English, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01


History, 21.10.2020 20:01

Social Studies, 21.10.2020 20:01



Mathematics, 21.10.2020 20:01




Mathematics, 21.10.2020 20:01

English, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01

History, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01



Arts, 21.10.2020 20:01