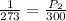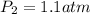Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal container cannot be changed. what will happen to the pressure if the temperature rises to 300k? a. it will fall to 0.7 atm. b. it will fall to 0.9 atm. c. it will rise to 1.1 atm. d. it will rise to 1.3 atm.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Neon gas occupies a metal container of 5.5 l at stp (273 k, 1 atm). the volume of the metal containe...
Questions






Mathematics, 05.09.2020 23:01



Computers and Technology, 05.09.2020 23:01

Social Studies, 05.09.2020 23:01



Mathematics, 05.09.2020 23:01

Mathematics, 05.09.2020 23:01




Mathematics, 05.09.2020 23:01

Mathematics, 05.09.2020 23:01

Mathematics, 05.09.2020 23:01

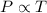 (At constant volume and number of moles)
(At constant volume and number of moles)
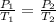
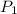 = initial pressure of gas = 1 atm
= initial pressure of gas = 1 atm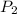 = final pressure of gas = ?
= final pressure of gas = ?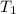 = initial temperature of gas =
= initial temperature of gas = 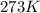
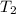 = final temperature of gas = 300 K
= final temperature of gas = 300 K