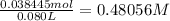Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2


Chemistry, 23.06.2019 21:30
This element is in the same family aa lead and it has fewer protons than sodium.
Answers: 2

Chemistry, 23.06.2019 22:30
Susann makes the following entry in her notebook on friday we are given a blue liquid in a shallow container we place it on the windowsill over the weekend or monday morning there was no liquid left with the dish had some solid blue stuff in it
Answers: 2
You know the right answer?
Nitric acid + mg(no3)35.0 ml of 0.255 m nitric acid is added to 45.0 ml of 0.328 m mg(no3)2. what is...
Questions



Mathematics, 07.02.2021 23:10


World Languages, 07.02.2021 23:10

Mathematics, 07.02.2021 23:10

Mathematics, 07.02.2021 23:10

Health, 07.02.2021 23:10


Health, 07.02.2021 23:10


History, 07.02.2021 23:10


History, 07.02.2021 23:10



Physics, 07.02.2021 23:10

Biology, 07.02.2021 23:10


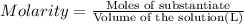
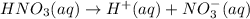
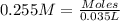
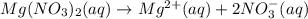
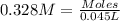
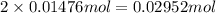 of nitrate ions
of nitrate ions