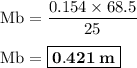Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
By titration it is found that 68.5 ml of 0.154 m naoh(aq) is needed to neutralize 25.0 ml of hcl(aq)...
Questions

Mathematics, 06.10.2019 02:30

History, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30


Mathematics, 06.10.2019 02:30

Social Studies, 06.10.2019 02:30





Mathematics, 06.10.2019 02:30



Mathematics, 06.10.2019 02:30

English, 06.10.2019 02:30

History, 06.10.2019 02:30

Physics, 06.10.2019 02:30



Mathematics, 06.10.2019 02:30