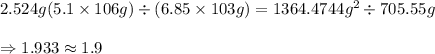Chemistry, 02.10.2019 14:10 PaMuth1100
2.524 g (5.1 × 106 g) ÷ (6.85 × 103 g) = ? how many significant figures should the result have?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
2.524 g (5.1 × 106 g) ÷ (6.85 × 103 g) = ? how many significant figures should the result have?...
Questions





History, 02.10.2020 19:01


Mathematics, 02.10.2020 19:01








Mathematics, 02.10.2020 19:01


Mathematics, 02.10.2020 19:01