
Chemistry, 20.09.2019 14:00 masonprice
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the reaction below. what is the concentration of the hcl? ca(oh)2(s) + 2hcl(aq) --> cacl2(aq) + h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 10:00
What is the density, d, of a substance with a volume of v = 12.5 cm3 and a mass of m = 74.4 g ?
Answers: 1

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2

Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1
You know the right answer?
A0.00100 mol sample of ca(oh)2 requires 25.00 ml of aqueous hcl for neutralization according to the...
Questions





Mathematics, 31.08.2020 19:01



Computers and Technology, 31.08.2020 19:01

Mathematics, 31.08.2020 19:01


History, 31.08.2020 19:01


Mathematics, 31.08.2020 19:01





Spanish, 31.08.2020 19:01

Spanish, 31.08.2020 19:01

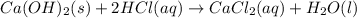
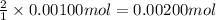
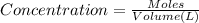
![[HCl]=\frac{0.00200 mol}{0.025 L}=0.08 mol/L](/tpl/images/0246/4244/941e7.png)


