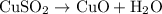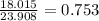Chemistry, 16.09.2019 18:40 juicemankinnie95
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cuo (mw = 79.545 g/mol) produced. given that 3.327 g of cuo was produced during the reaction, how many grams of water were released as water vapor? (number of moles = mass (g) / molar mass (g/ choose the closest answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
In the decomposition reaction, 1 mole of water (mw = 18.015 g/mol) was produced for every mole of cu...
Questions


Chemistry, 12.10.2020 17:01



English, 12.10.2020 17:01

History, 12.10.2020 17:01


Mathematics, 12.10.2020 17:01


Law, 12.10.2020 17:01

Mathematics, 12.10.2020 17:01


Business, 12.10.2020 17:01

Mathematics, 12.10.2020 17:01

Arts, 12.10.2020 17:01




Mathematics, 12.10.2020 17:01

History, 12.10.2020 17:01