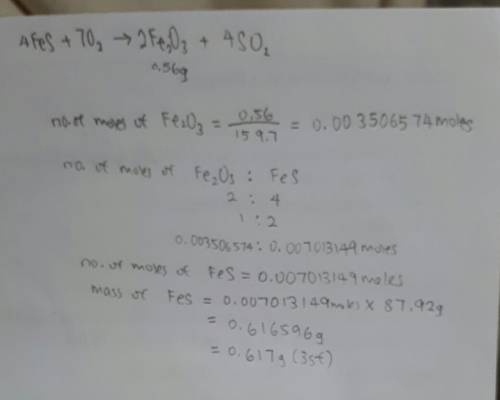In the following reaction, how many grams of ferrous sulfide (fes) will produce
0.56 grams of...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1


Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Questions

Chemistry, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01



Chemistry, 12.10.2020 21:01

English, 12.10.2020 21:01





Biology, 12.10.2020 21:01



History, 12.10.2020 21:01


Biology, 12.10.2020 21:01

English, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01