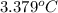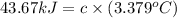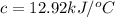Chemistry, 26.09.2019 22:40 kraigstlistt
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol. when 1.411 g of compound a (molar mass = 115.27 g/mol) was burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.379 °
c. using this data, what is the heat capacity (calorimeter constant) of the calorimeter?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is –3568.0 kj/mol....
Questions





Advanced Placement (AP), 13.06.2020 03:57















Mathematics, 13.06.2020 03:57

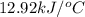
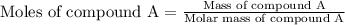
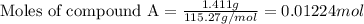
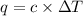
 = change in temperature =
= change in temperature =