

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Give the theoretical yield, in moles, of co2 from the reaction of 4.00 moles of c8h18 with 4.00 mole...
Questions

Biology, 09.06.2020 22:57


Mathematics, 09.06.2020 22:57

Biology, 09.06.2020 22:57


Mathematics, 09.06.2020 22:57

Mathematics, 09.06.2020 22:57

Mathematics, 09.06.2020 22:57

Mathematics, 09.06.2020 22:57



Computers and Technology, 09.06.2020 22:57

English, 09.06.2020 22:57


Mathematics, 09.06.2020 22:57


Mathematics, 09.06.2020 22:57

Chemistry, 09.06.2020 22:57

Mathematics, 09.06.2020 22:57

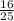 x 4 ) moles of CO₂
x 4 ) moles of CO₂ 

