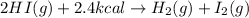Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
Determine the value for the following reaction. 2hi(g) + 2.4 kcal → h2(g) + l2(g) δh =...
Questions


Biology, 29.04.2021 18:20


Mathematics, 29.04.2021 18:20



Mathematics, 29.04.2021 18:20



Health, 29.04.2021 18:20


Mathematics, 29.04.2021 18:20

Mathematics, 29.04.2021 18:20

History, 29.04.2021 18:20


Mathematics, 29.04.2021 18:20

Biology, 29.04.2021 18:20


Mathematics, 29.04.2021 18:20

English, 29.04.2021 18:20

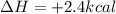
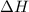 for the reaction comes out to be positive.
for the reaction comes out to be positive.