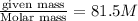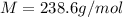Chemistry, 31.08.2019 08:10 luximartinez
What is the molar mass of 81.50g of gas exerting a pressure of 1.75atm on the walls of a 4.92l container at 307k?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
What is the molar mass of 81.50g of gas exerting a pressure of 1.75atm on the walls of a 4.92l conta...
Questions


Mathematics, 29.07.2019 22:30

Biology, 29.07.2019 22:30

Mathematics, 29.07.2019 22:30

History, 29.07.2019 22:30

History, 29.07.2019 22:30

English, 29.07.2019 22:30


Mathematics, 29.07.2019 22:30



Mathematics, 29.07.2019 22:30

Mathematics, 29.07.2019 22:30


English, 29.07.2019 22:30



Chemistry, 29.07.2019 22:30

Mathematics, 29.07.2019 22:30

Mathematics, 29.07.2019 22:30