By examining electron domain geometry, one can determine that the ammonia molecule (nh3) has
...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1
You know the right answer?
Questions


Mathematics, 20.09.2020 06:01


Computers and Technology, 20.09.2020 06:01




Computers and Technology, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01



Mathematics, 20.09.2020 06:01





Computers and Technology, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

![\text{Number of electron pairs}=\frac{1}{2}[V+N-C+A]](/tpl/images/0402/1365/0b8ba.png)
![\text{Number of electron pairs}=\frac{1}{2}[5+3-0+0]=4](/tpl/images/0402/1365/f374b.png)
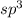 .
.

