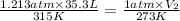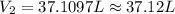Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Asample of propane, a component of lp gas, has a volume of 35.3 l at 315 k and 922 torr. what is its...
Questions

Mathematics, 19.01.2020 10:31

Mathematics, 19.01.2020 10:31


Geography, 19.01.2020 10:31

Computers and Technology, 19.01.2020 10:31

Mathematics, 19.01.2020 10:31


History, 19.01.2020 10:31




English, 19.01.2020 10:31

History, 19.01.2020 10:31




Mathematics, 19.01.2020 10:31

Biology, 19.01.2020 10:31

Mathematics, 19.01.2020 10:31

History, 19.01.2020 10:31

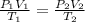
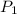 = initial pressure of gas = 922 torr = 1.213 atm
= initial pressure of gas = 922 torr = 1.213 atm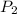 = final pressure of gas at STP = 1 atm
= final pressure of gas at STP = 1 atm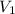 = initial volume of gas = 35.3 L
= initial volume of gas = 35.3 L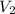 = final volume of gas at STP = ?
= final volume of gas at STP = ?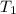 = initial temperature of gas = 315 K
= initial temperature of gas = 315 K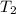 = final temperature of gas at STP = 273 K
= final temperature of gas at STP = 273 K