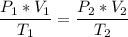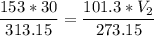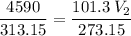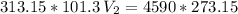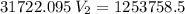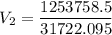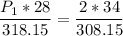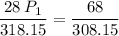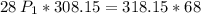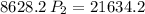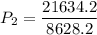Chemistry, 05.10.2019 09:01 zhellyyyyy
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the balloon have at standard temperature and pressure (273.15 k and 101.3 kpa)?
a. 17.3 l
b. 23.7 l
c. 39.5 l
d. 51.9 l
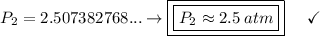
a gas that has a volume of 28 liters, a temperature of 45 °c, and an unknown pressure, has its volume increased to 34 liters and its temperature decreased to 35 °c. if i measure the pressure after the change to be 2.0 atm, what was the original pressure of the gas?
a. 1.5 atm
b. 1.7 atm
c. 2.8 atm
d. 2.5 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

You know the right answer?
The volume of a gas-filled balloon is 30.0 l at 40 °c and 153 kpa pressure. what volume will the bal...
Questions








Geography, 10.07.2021 04:10

Mathematics, 10.07.2021 04:10

Mathematics, 10.07.2021 04:20

Mathematics, 10.07.2021 04:20


Mathematics, 10.07.2021 04:20