Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

You know the right answer?
Amixture of two gases exerts a total pressure of 2.25 atm. if the partial pressure of one of the gas...
Questions


Health, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

History, 11.10.2020 14:01

English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01




Social Studies, 11.10.2020 14:01




History, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01


English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

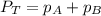
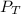 = total partial pressure = 2.25 atm
= total partial pressure = 2.25 atm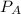 = partial pressure of one gas = 1.43 atm
= partial pressure of one gas = 1.43 atm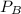 = partial pressure of another gas = ?
= partial pressure of another gas = ?