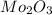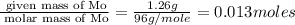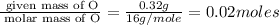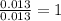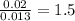Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybdenum over a bunsen burner. the following data was collected:
mass of crucible: 38.26 g
mass of crucible and molybdenum: 39.52 g
mass of crucible and molybdenum oxide: 39.84 g
solve for the empirical formula of the compound, showing your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Acompound that is composed of molybdenum (mo) and oxygen (o) was produced in a lab by heating molybd...
Questions

History, 18.09.2021 01:50



History, 18.09.2021 01:50

Mathematics, 18.09.2021 01:50





Mathematics, 18.09.2021 01:50






Mathematics, 18.09.2021 01:50


Mathematics, 18.09.2021 01:50

Computers and Technology, 18.09.2021 01:50

History, 18.09.2021 01:50