
Chemistry, 06.10.2019 12:20 destinywiggins75
Which is a characteristic of weak acids and weak bases?
a. ka and kb values of weak acids and weak bases are small.
b. forward reactions of weak acids and weak bases are favored.
c. dissociation of weak acids and weak bases is nearly complete.
d. many h+ or oh– ions are present in their solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Which is a characteristic of weak acids and weak bases?
a. ka and kb values of weak acids and...
a. ka and kb values of weak acids and...
Questions


History, 06.12.2020 02:40


Physics, 06.12.2020 02:40


Biology, 06.12.2020 02:40

Mathematics, 06.12.2020 02:40




Mathematics, 06.12.2020 02:40


Mathematics, 06.12.2020 02:40


Mathematics, 06.12.2020 02:40

Business, 06.12.2020 02:40

Chemistry, 06.12.2020 02:40

Arts, 06.12.2020 02:40


Arts, 06.12.2020 02:40

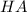 ⇔
⇔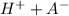
![K_{a}=\frac {[H^{+}][A^{-}]}{[HA]}](/tpl/images/0293/5761/dc648.png)
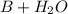 ⇔
⇔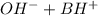
![K_{b}=\frac {[OH^{-}][BH^{+}]}{[B]}](/tpl/images/0293/5761/e6d15.png)


