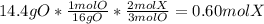Chemistry, 09.10.2019 15:30 recon12759
The 30.6-g sample of the compound x2o3 contains 14.4 g of oxygen atoms. what is the molar mass of element x?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1


You know the right answer?
The 30.6-g sample of the compound x2o3 contains 14.4 g of oxygen atoms. what is the molar mass of el...
Questions





Computers and Technology, 12.03.2020 04:54








Social Studies, 12.03.2020 04:56






Mathematics, 12.03.2020 04:56