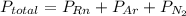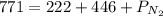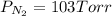Chemistry, 31.08.2019 11:00 kirsten8605
Agas cylinder contains only the gases radon, nitrogen, and helium. the radon has a pressure of 222 torr while the nitrogen has a pressure of 446 torr. if the total pressure inside the cylinder is 771 torr, what is the pressure that is due to the helium? a gas cylinder contains only the gases radon, nitrogen, and helium. the radon has a pressure of 222 torr while the nitrogen has a pressure of 446 torr. if the total pressure inside the cylinder is 771 torr, what is the pressure that is due to the helium? 549 torr 668 torr 103 torr 771 torr none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

You know the right answer?
Agas cylinder contains only the gases radon, nitrogen, and helium. the radon has a pressure of 222 t...
Questions

Biology, 26.07.2019 01:40

English, 26.07.2019 01:40

History, 26.07.2019 01:40



History, 26.07.2019 01:40




Arts, 26.07.2019 01:40



English, 26.07.2019 01:50

History, 26.07.2019 01:50



Spanish, 26.07.2019 01:50


Social Studies, 26.07.2019 01:50