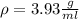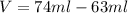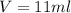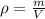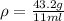Chemistry, 18.09.2019 21:00 miguelelmenor910
The philosopher’s stone weighs 43.2 g is placed in a graduated cylinder containing 63 ml of water. after the stone is added to the cylinder the water rises to 74 ml. what is the density of the stone? express your answer in g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

You know the right answer?
The philosopher’s stone weighs 43.2 g is placed in a graduated cylinder containing 63 ml of water. a...
Questions





Engineering, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40


History, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

Mathematics, 06.01.2021 01:40

English, 06.01.2021 01:40

Computers and Technology, 06.01.2021 01:40




English, 06.01.2021 01:40

Geography, 06.01.2021 01:40